AK treatment drug potential “game-changer”

Professor John Dart
New clinical trial results with a drug to treat sight-threatening Acanthamoeba keratitis (AK) have been described as potentially “game-changing” by renowned AK expert and study lead, Professor John Dart.
The drug, polihexanide, is based on UCL and Moorfields Eye Hospital research and is currently under development by Italian ophthalmic pharmaceuticals firm, Sifi. It has been found to be highly effective in treating AK in a new international clinical trial – with the findings published in Ophthalmology.
Low concentration polihexanide (PHMB 0.02 per cent) was first compounded and used in the 1990s to treat AK, introduced by a team co-led by Professor Dart. It is widely recommended as a treatment for AK, but it is not a licensed drug, and treatment outcomes have been variable.
Professor Dart, from UCL Institute of Ophthalmology and Moorfields Eye Hospital NHS Foundation Trust, said: “Acanthamoeba keratitis in contact lens users can be prevented by following safe use advice: do use daily disposables if possible, wash and dry hands before handling lenses, maintain good lens and lens case hygiene, and don’t use them when bathing swimming or showering, or use goggles and renew the lens after use, don’t wear them overnight, and don’t use them every day. Unfortunately, when the disease does develop, the course is prolonged and, in the recent past, one third of patients have had poor visual outcomes with one quarter requiring surgery at some stage.
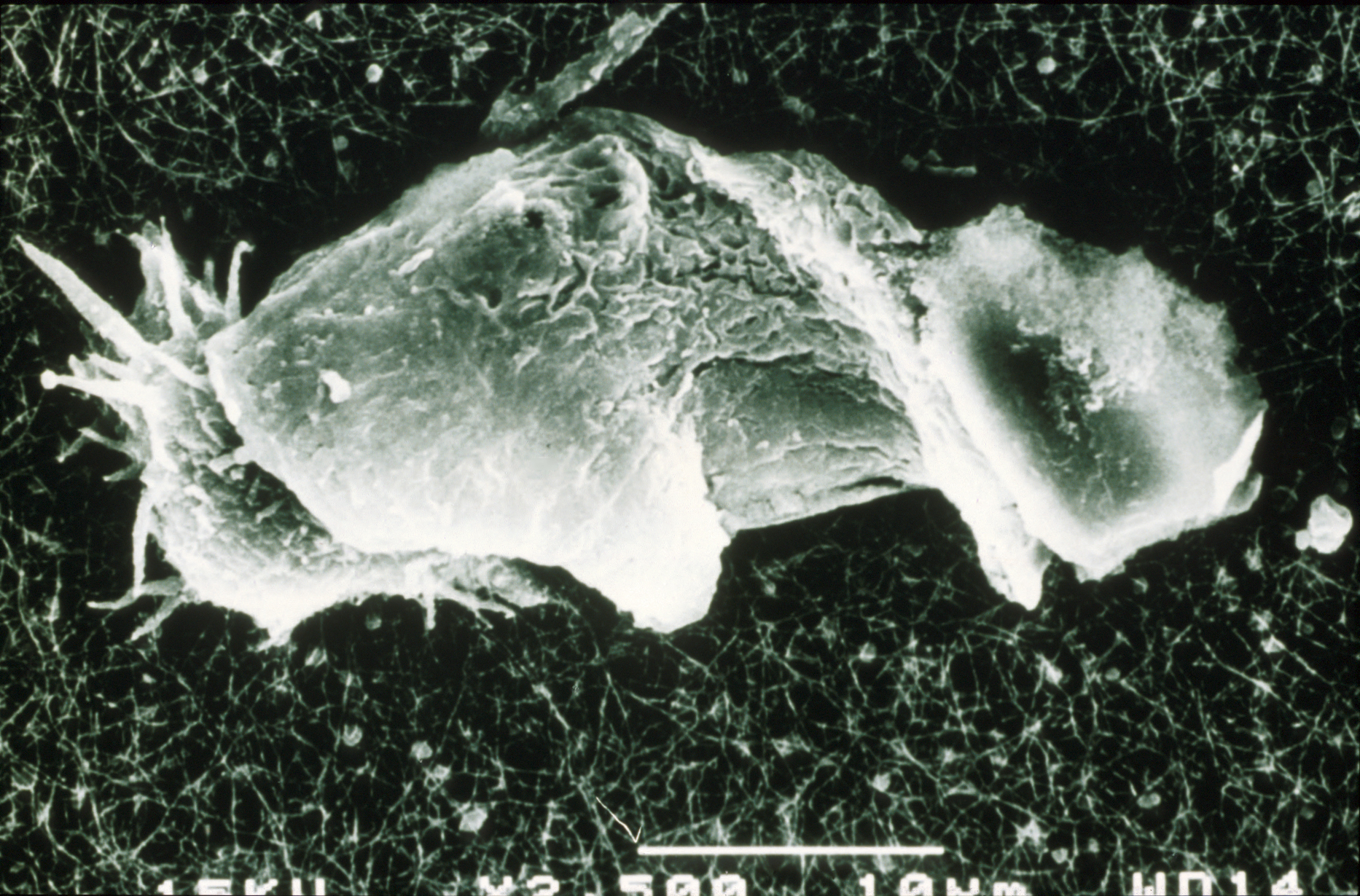
Electron microscope scan of a cyst from AK infection
“PHMB 0.02 per cent is an effective and widely recommended unlicensed therapy, but many clinicians have trouble accessing it; mistakes in formulation can sometimes lead to poor results, and the lack of a proven treatment protocol has resulted in wide variations in how the drug is used and in treatment outcomes. We hope that our new robust findings with polihexanide 0.08 per cent will be a game changer for AK treatment, by improving access and the consistency of treatment, addressing currently unmet patient needs.”
The researchers stated that the widely recommended dual therapy was more effective than usual in this trial because clinicians were strictly following a set treatment protocol. In addition, they said, the new monotherapy has advantages over dual therapy, “as the simplicity reduces the risk of errors in practice”.
Dr Vincenzo Papa, head of scientific affairs at Sifi and co-author of the study, said: “This publication in Ophthalmology, the leading peer-reviewed journal in our field, further encourages our continued efforts to make polihexanide 0.08 per cent [Akantior] available to patients with AK, as the first approved orphan medicinal product. Given the extreme burden of the disease and the high unmet medical need, we are proud of the high efficacy outcomes in the robust setting that the trial created, especially when compared with the efficacy rates of 60 per cent reported with the current best treatment.”
Sifi is seeking regulatory approvals for polihexanide 0.08 per cent in Europe, the UK and the US.
Read more about the study and AK here.